
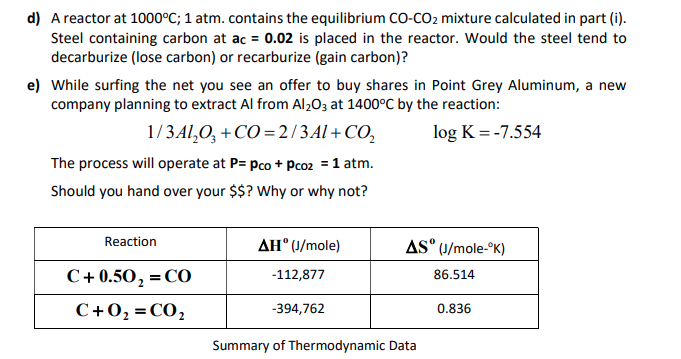
In the reaction C(s) + CO2(g) 2CO(g) the equilibrium pressure is 12 atm. If 50% of CO2 reacts calculate Kp .

For the reaction: C (s)+ CO2 (g) 2CO (g) the partial pressure of CO2 and CO are 2 and 4 atm respectively at equilibrium. Then equilibrium costant for the reaction is -
3.For the equilibrium C(s)+ COg) gives 2CO(g) Kp is 63 atm at 1000 K. If at equilibrium Pco=10Pco2 then total pressure at equilibrium is : 4.A(g) is 90

Solve this: â ‹Q3 For the equilibrium C(s) + CO2(g) ⇌2CO(g) KP = 63 atm at 1000 K - Chemistry - Chemistry in Everyday Life - 11997217 | Meritnation.com

CH4 and CO2 conversions in the reaction of DRM (CH4 + CO2 → 2H2 + 2CO)... | Download Scientific Diagram
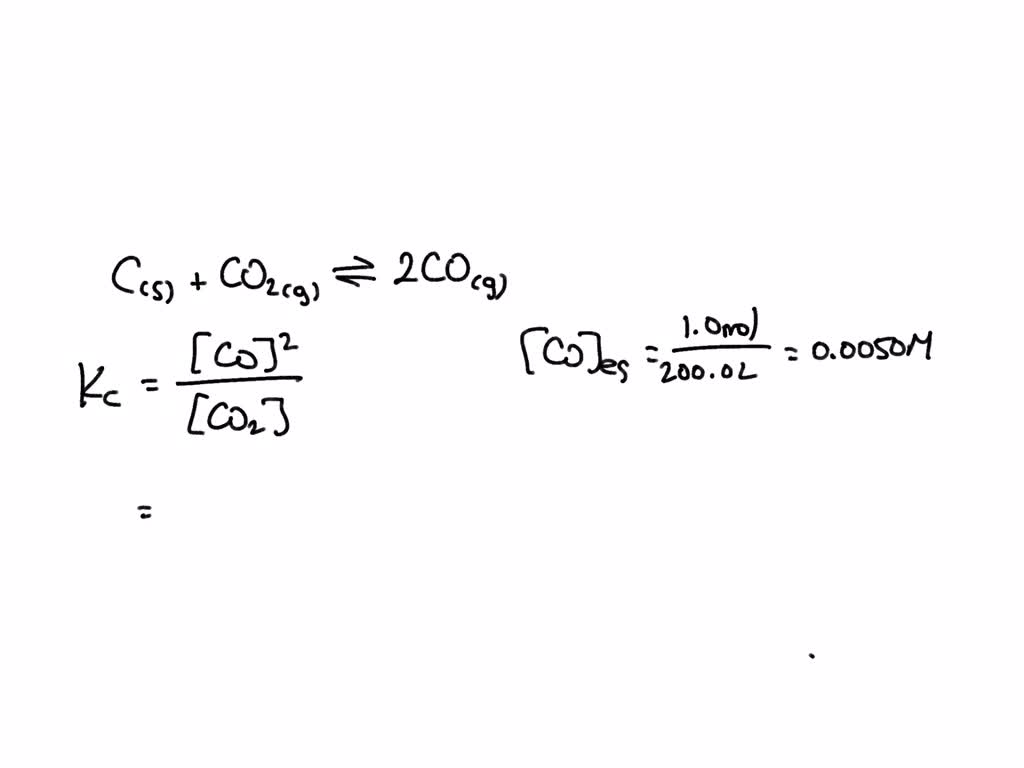
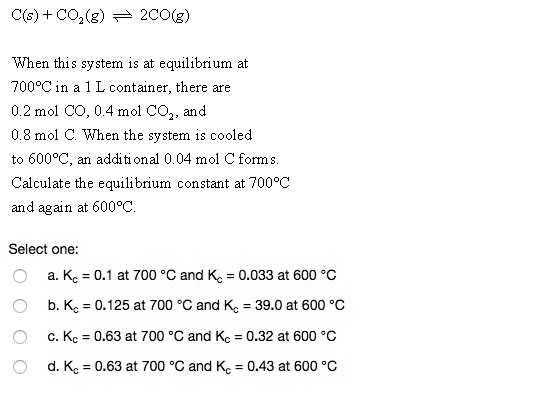
SOLVED: The reaction C(s) + CO2(g) ⇔ 2CO(g) occurs at high temperatures. At 700°C, a 200.0 L tank contains 1.0 mol CO, 0.20 mol CO2, and 0.40 mol of C at equilibrium.

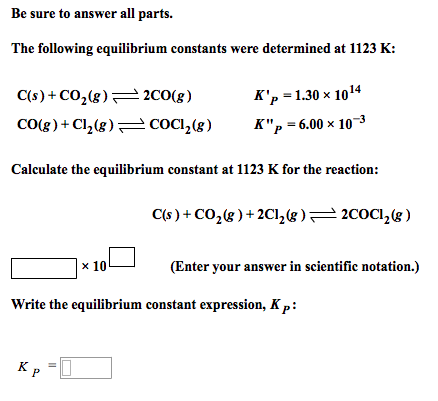
Given, 2CO(g) C(s) + CO2(g); Kp1 = 10^-14atm^-1 at 1120 K CO(g) + Cl2(g) COCl2(g); Kp2 = 6 × 10^-3 atm^-1 The value of Kc for the following reaction at 1120 K

Equilibrium concentrations of reactants and products in the reaction C... | Download Scientific Diagram

For the reaction, C (s) + CO2 (g) 2CO (g) , the partial pressures of CO2 and CO are 2.0 and 4.0 atm respectively at equilibrium. The Kp for the reaction is:








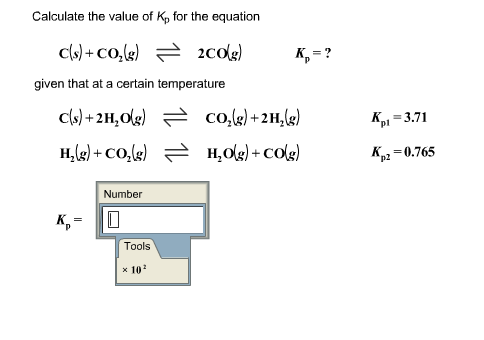



![ANSWERED] K is 7.7x10-15 for the reaction 2CO(g)=C(s... - Organic Chemistry ANSWERED] K is 7.7x10-15 for the reaction 2CO(g)=C(s... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/53142838-1659042473.5846057.jpeg)
