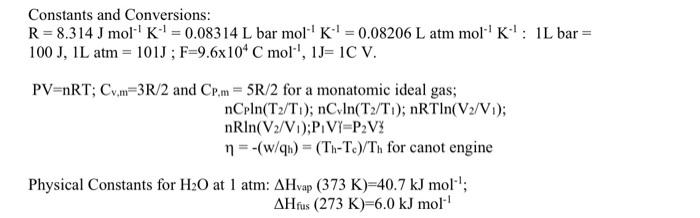
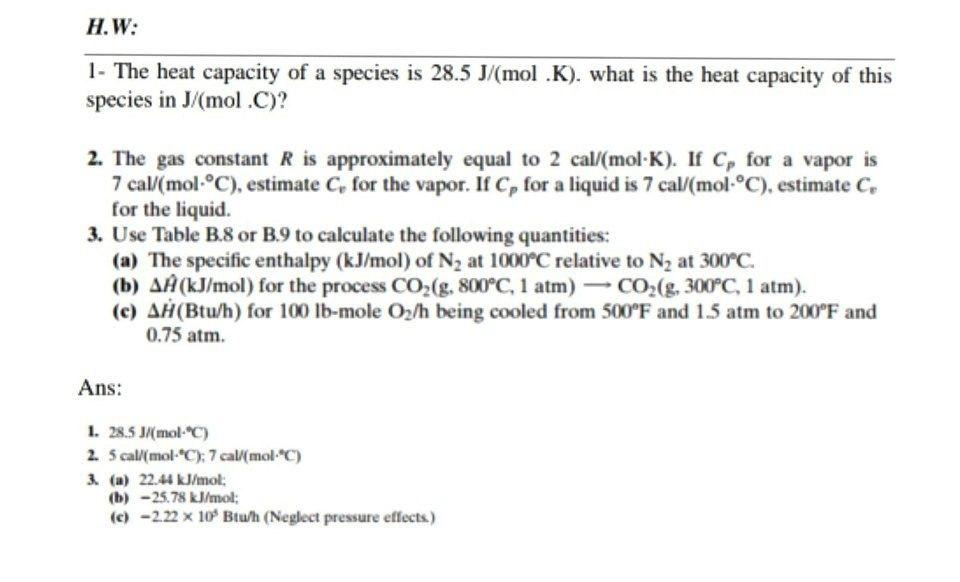
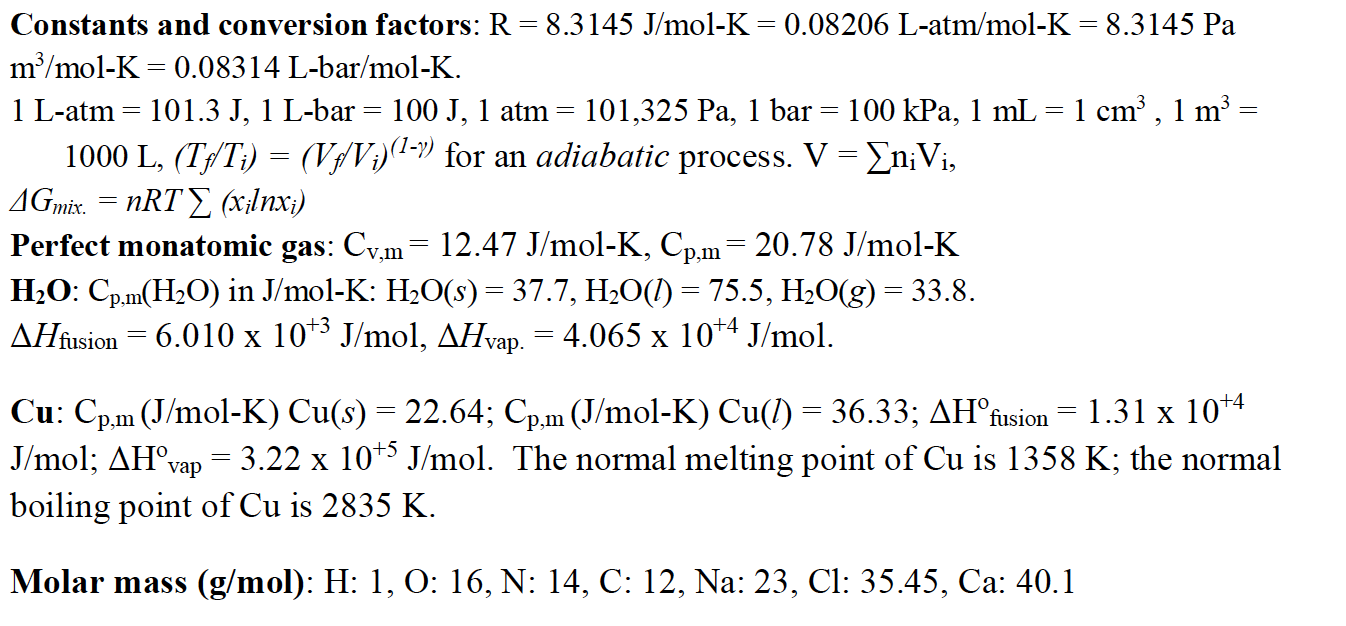
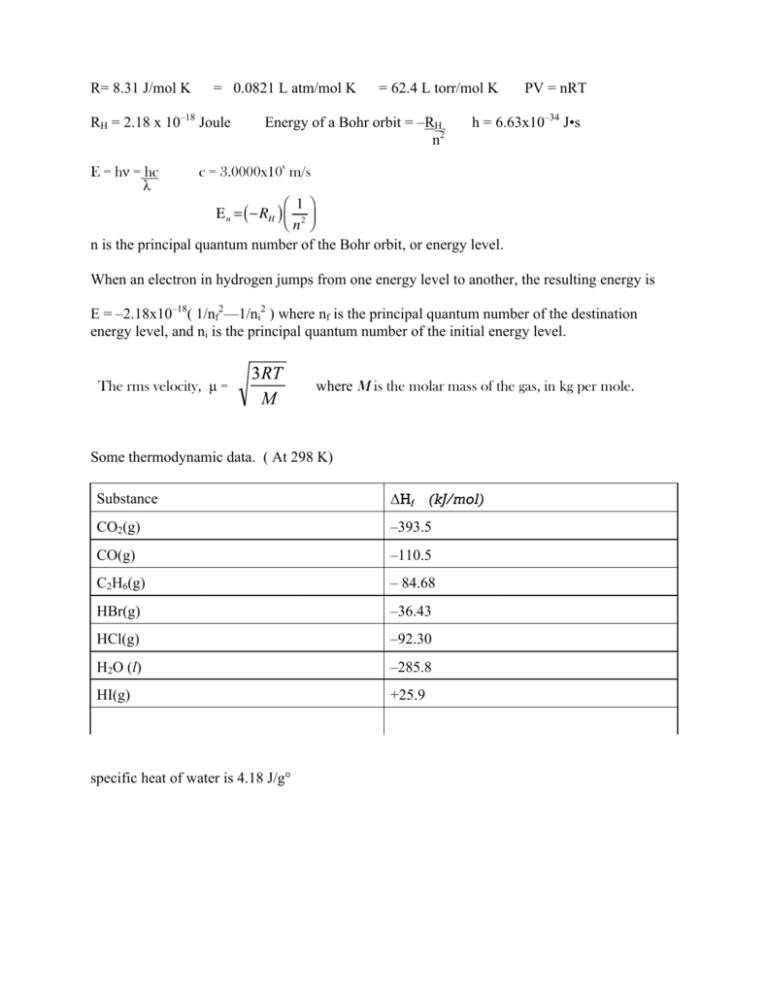
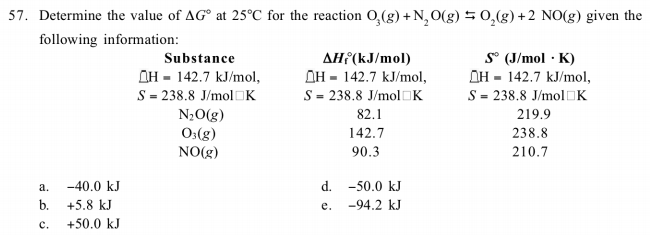
58.03 kj/ mole at 0C Cpof liquid water = 75.3j/mol/k Cpof solidvwater = 36.8 jmol//Calculate the enthalpy on freezing of 1 mole of water at 10*C to ice at 10*C enthalpy of fus. 6.

Two moles of helium gas is mixed with three moles of hydrogen molecules (taken to be rigid). What is the molar specific heat of mixture at constant volume ? (R = 8.3
36.) Calculate the rms speed of an ideal diatomic gas having molecular weight 32 gm/mol at Oc If the specific heats at constant pressure and volume are respectively 29.1 J mol1 K

Three moles of an ideal gas are taken around the cycle abc shown in the figure. For this gas, C_ p = 29.1\ \frac{J}{mol.K}. Process ac is at constant pressure, process ba
![ANSWERED] What is the internal energy of 7.00 mol o... - Physical Chemistry - Kunduz ANSWERED] What is the internal energy of 7.00 mol o... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question/raw/52923887-1659262298.2603226.jpeg)