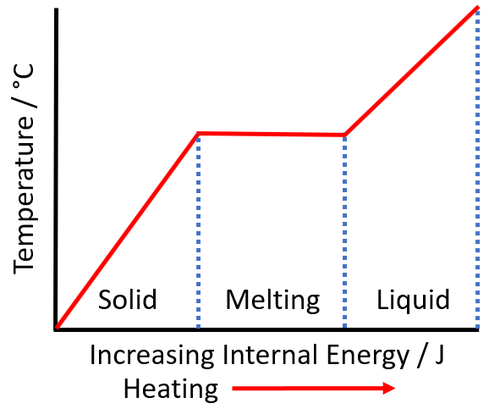
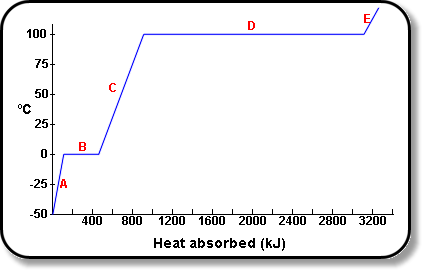
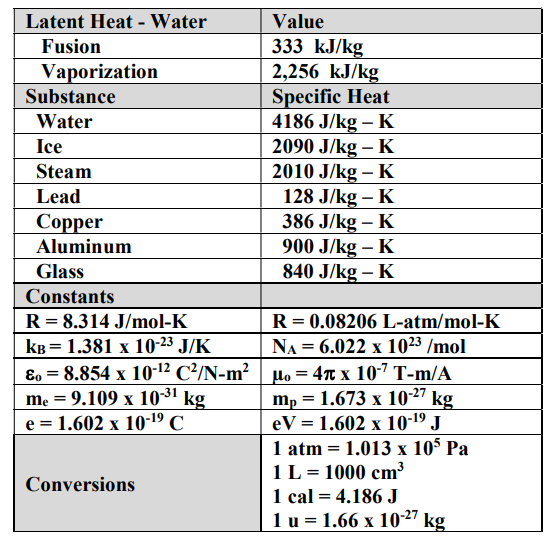
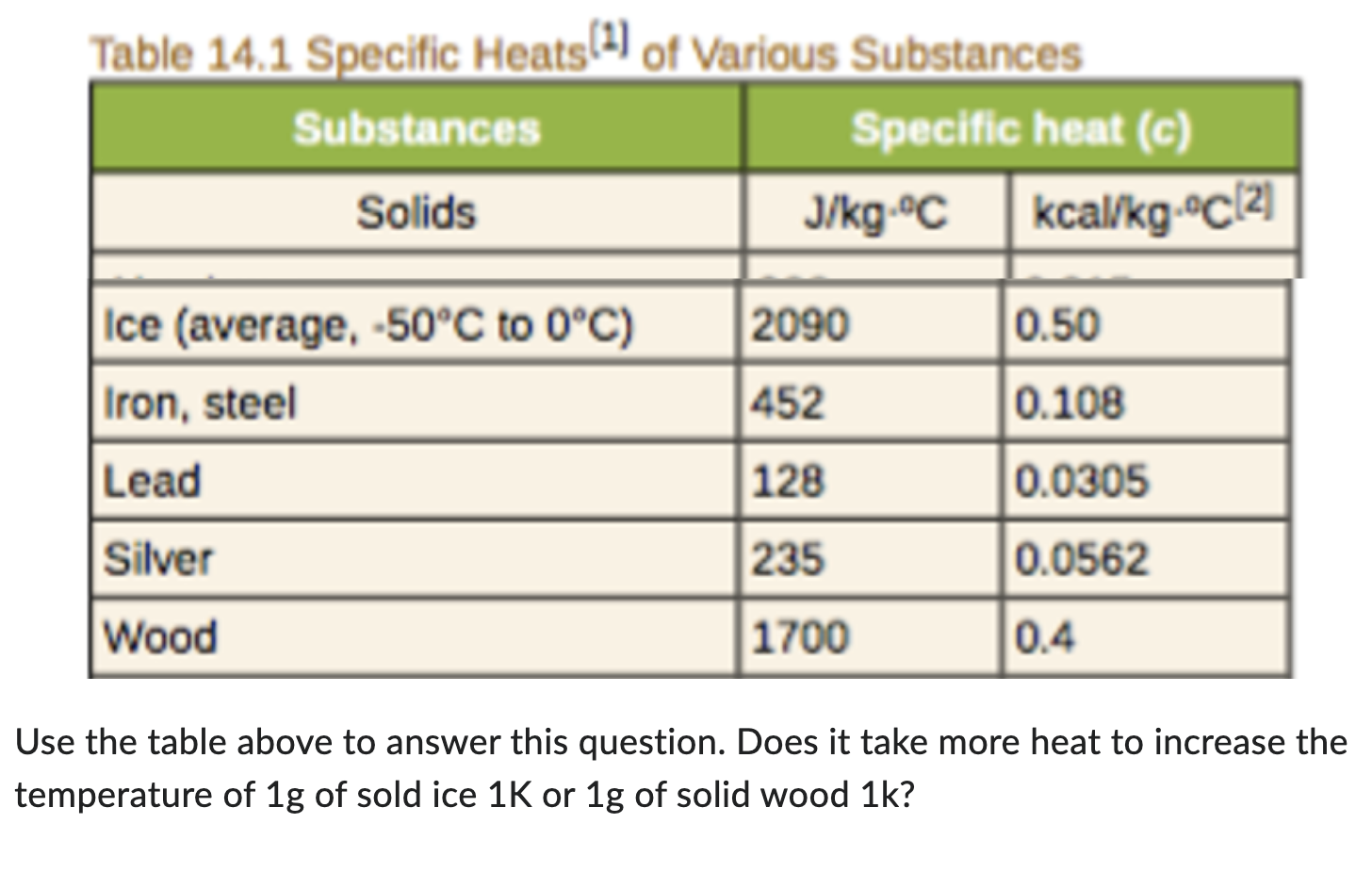
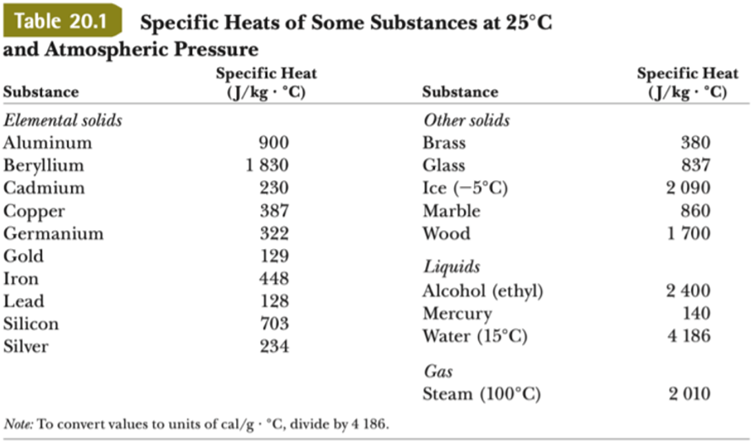
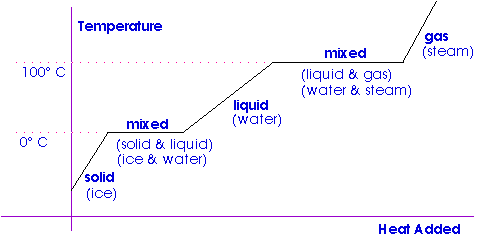
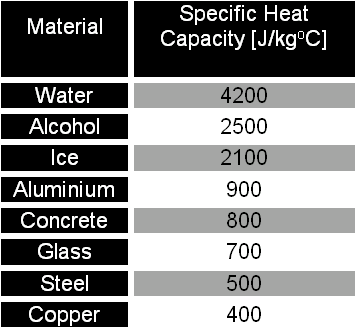
1 PHYS1001 Physics 1 REGULAR Module 2 Thermal Physics HEAT CAPACITY LATENT HEAT What is cooking all about? ptC_heat.ppt. - ppt download
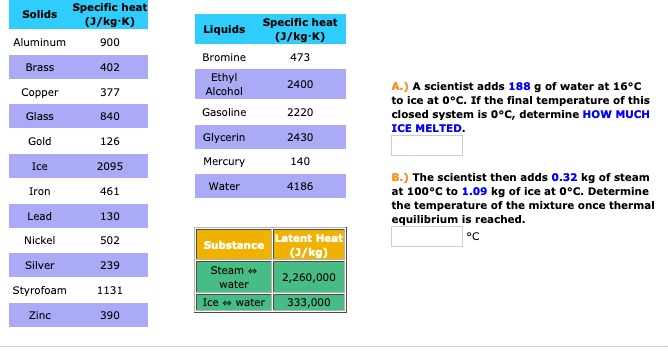
SOLVED: Specific heat Solids (J/kg K) Specific heat Liquids (J/kg K) Bromine 473 Ethyl Alcohol 2400 Aluminum 900 Brass 402 Copper 377 Glass 840 Gasoline 2220 Gold 126 Glycerin 2430 Mercury 140
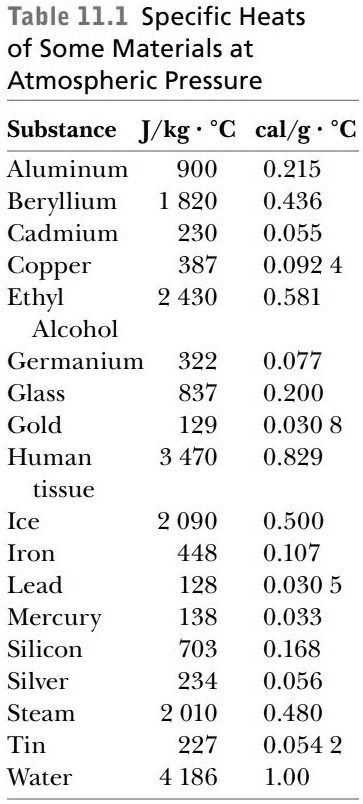
SOLVED: Table 11.1 Specific Heats of Some Materials at Atmospheric Pressure Substance J/kg 'C cal/g Aluminum 900 0.215 Beryllium 820 0.436 Cadmium 230 0.055 Copper 387 0.092 4 Ethyl 2 430 0.581
Heat energy of 184 kJ is given to ice of mass 600 g at -12°C, Specific heat of ice is 2222.3 J - Sarthaks eConnect | Largest Online Education Community

a) Specific heat capacity c (Eq. (11)), thermal conductivity k (Eq.... | Download Scientific Diagram

The amount of heat energy required to convert 1 kg of ice at - 10^∘C to water at 100^∘C is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat capacity

SOLVED: Specific heat capacity of water = 4186 J kg^-1 K^-1 Specific heat capacity of ice = 2090 J kg^-1 K^-1 Latent heat of fusion of ice = 335 kJ kg^-1 Latent
![Specific heat capacity C p [J/(kg·K)] of small samples (E, F, and G) at... | Download Scientific Diagram Specific heat capacity C p [J/(kg·K)] of small samples (E, F, and G) at... | Download Scientific Diagram](https://www.researchgate.net/publication/256115072/figure/tbl1/AS:755605098729495@1557161702871/Specific-heat-capacity-C-p-J-kgK-of-small-samples-E-F-and-G-at-various.png)
Specific heat capacity C p [J/(kg·K)] of small samples (E, F, and G) at... | Download Scientific Diagram
What is the difference between specific latent heat of melting of ice and specific latent heat of fusion of ice? - Quora